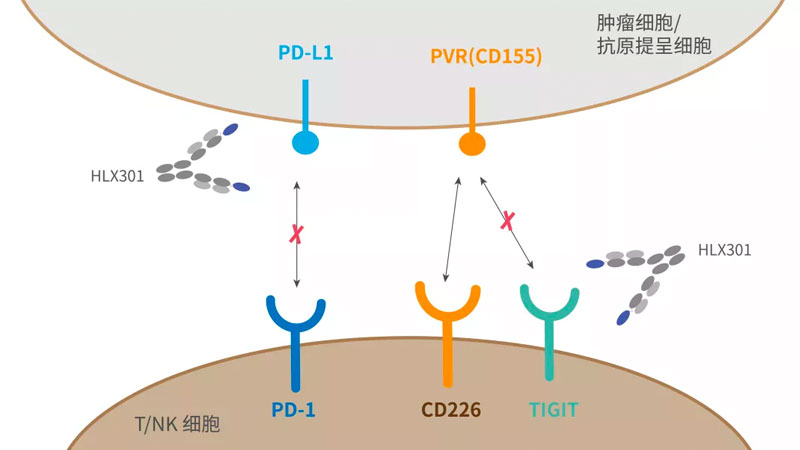
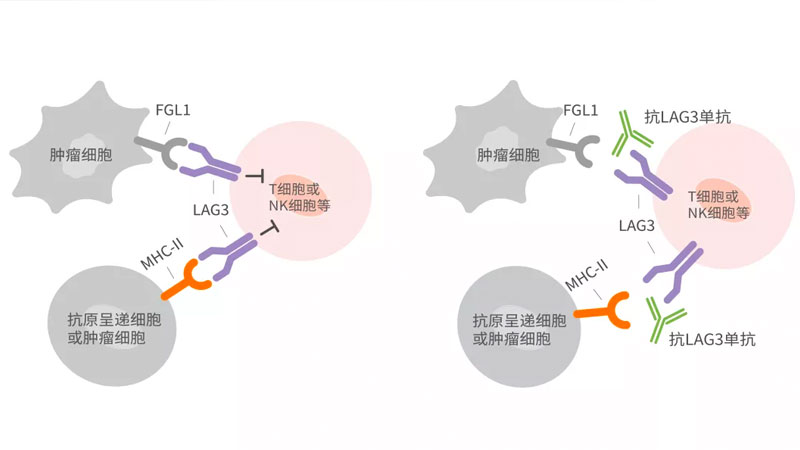
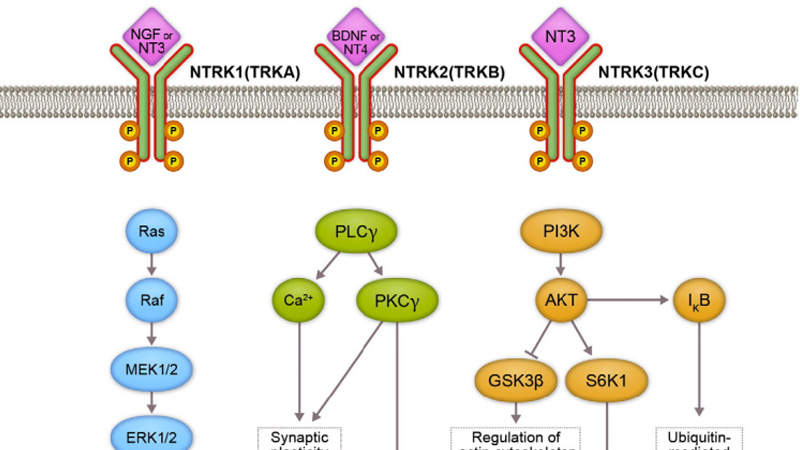
-
Our Company
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China

- Fosun Pharma is patient-centered and clinical needs-oriented. The company continuously enriches its innovative product pipeline through independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
Our Business
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (Fosun Pharma) directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.

- Fosun Pharma is patient-centered and clinical needs-oriented. The company enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma has formed technological platforms for innovative small molecule drugs, antibody drugs, and cell therapy with a focus on key disease areas including oncology and immunomodulation, metabolism and digestive system, and central nervous system. Fosun Pharma also vigorously explores cutting-edge technologies, such as RNA, oncolytic viruses, gene therapy and PROTAC, to enhance its innovation capabilities.
-
R&D
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Guided by innovation and internationalization, the company continuously enriches its innovative product pipeline through diversified and multi-level cooperation models such as independent research and development, cooperative development, license-in, and in-depth incubation. Fosun Pharma improves the research and clinical development capabilities of FIC (First-in-class) and BIC (Best-in-class) new drugs as well as accelerates the R&D and launch of innovative technologies and products.
-
Sustainability
In pursuit of sustainable development of talents and products is the social responsibility concept of Fosun Pharma.

- Fosun Pharma has incorporated sustainable development into the Group's overall development strategy, emphasizing long-term ESG governance capabilities, and established a three-level ESG governance structure supervised by the board of directors (the “Board”), implemented by the ESG Committee, and executed by the ESG Working Group. The ESG Committee of the Board is responsible for formulating and promoting the Group's ESG vision, goals, and strategies, and provides advice to the Board; the ESG Working Group is responsible for sorting out key ESG issues, formulating sustainable development quantitative targets, and tracking progress. The ESG Committee and the ESG Working Group are committed to integrating ESG concepts into corporate operations and enhancing the company's sustainability.
-
Investor Relations
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196.SH, 02196.HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Group Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
News
Shanghai Fosun Pharmaceutical (Group) Co., Ltd. is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China.

- Founded in 1994, Shanghai Fosun Pharmaceutical (Group) Co., Ltd. ("Fosun Pharma"; stock code: 600196. SH, 02196. HK) is a global innovation-driven pharmaceutical and healthcare industry group deep-rooted in China. Fosun Pharma directly operates businesses including pharmaceutical manufacturing, medical devices, medical diagnosis, and healthcare services. As a shareholder of Sinopharm Co., Ltd., Fosun Pharma expands its areas in the pharmaceutical distribution and retail business.
-
Careers
Some top talent with strong entrepreneurship gathers here with us.

- We are looking for people that recognize and practice our cultural values, and can learn fast to create continuous value; possess profound professional knowledge of the industry, a wide vision of different regions, and global-level expertise in a certain field. We will also be committed to cultivating international top talent with outstanding performance and great potential.